Be the first to receive MyVeeva updates, including eConsent launching August 2021.
Providing you with control over the communications you receive from Veeva is important to us. Read our data privacy policy.
Sign up today!

Providing you with control over the communications you receive from Veeva is important to us. Read our data privacy policy.
Sign up today!

Join us to explore how a connected eConsent system can help sites provide a better patient experience and run more efficient clinical trials.

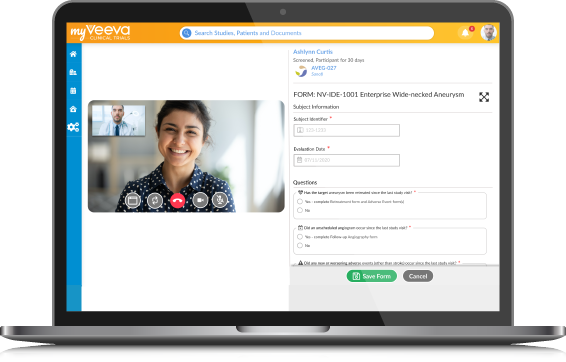
Crofoot Research Center electronically consented the first patient on Veeva eConsent, a MyVeeva for Patients application, for a phase 2b study.

Multichannel patient portal will help clinical research sites conduct virtual patient visits eSource capability will enable paperless clinical trials